Recent Landmark Medicines originated from Japan
- 1. Tacrolimus (PrografR; Fujisawa Pharmaceutical Co., Ltd.)
- 2. Pravastatin(MEVALOTINR ;Sankyo Co., Ltd.)
- 3. Donepezil (AriceptR; Eisai Co. Ltd.)
- 4. Levofloxacin (CravitR; Daiichi Pharmaceutical Co., Ltd.)
- 5. Leuprorelin (LeuplinR; Takeda Chemical Industries, Ltd.)
A novel immunosuppressant Tacrolimus hydrate from Streptomyces tukbaensis
Tacrolimus hydrate, a novel 23-membered macrolide, was discovered during extensive screening of fermentation products to identify a compound inhibiting the mixed lymphocyte reaction (MLR). The Streptomyces tsukubaensis that produces Tacrolimus hydrate was found near Mt.Tsukuba, Ibaraki, Japan, and Tacrolimus was isolated as prismatic crystals. In light of the high efficacy of Tacrolimus in transplantation in multicenter randomized clinical studies, Tacrolimus was marketed in 1993 in Japan and was followed in 1994 in the USA and is now available worldwide. Clinical studies to explore applications to autoimmune diseases, have led to the use of Tacrolimus ointment in atopic dermatitis, an indication for which it is marketed in Japan, the USA and the EU at present.
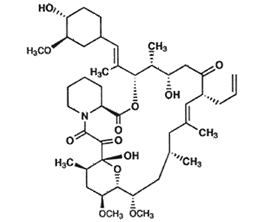
Structure of Tacrolimus (FK506)
Pravastatin, a cholesterol-lowering agent
High cholesterol level in blood is a risk factor for coronary heart disease and HMG-CoA reductase catalyzes the rate-limiting step of cholesterol biosynthesis. The benefit of treating hyperlipidemia has widely been accepted for reducing the risk. Pravastatin, an HMG-CoA reductase inhibitor, decreases blood cholesterol levels. Now pravastatin sodium (MEVALOTIN ®) is available in about 90 countries and regions around the world, contributing to the treatment of 4.5 million people with hyperlipidemia.
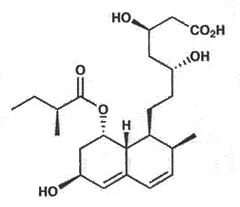
Chemical Structure of Pravastatin
Donepezil, an Alzheimer's Disease drug, improves QOL.
Dementia associated with aging has become a critical issue in developed countries,. Alzheimer's disease, the most common type of senile dementia, accounts for 50 to 60% of dementia patients. Although the cause of a progressive, neurodegenerative disease has not been elucidated yet, the level of neurotransmitter acetylcholine is very low in the brain of patients with Alzheimer's disease (acethylcholine hypothesis). Donepezil, a structurally and functionally new class of acetylcholinesterase inhibitor increases the level of acetylcholine in the brain, improves memory, cognition and global clinical function and thereby improves the Quality of Life (QOL) of Alzheimer's disease patients. This drug is the first choice in Alzheimer's disease treatment in over 60 countries worldwide. Proof of acethylcholine hypothesis by discovery of donepezil has lead to further progress of medical and pharmaceutical science and pharmaceutics.
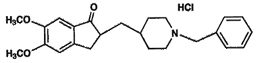
(+-)-2-[(1-benzylpiperidine-4-yl)methyl]
-5,6-dimethoxy-indan-1-one monohydrochloride
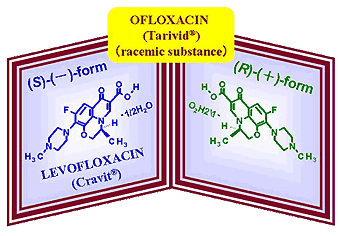
LEVOFLOXACIN (Cravit), the first chiral fluoroquinolone antibacterial agent
Ofloxacin (Tarivid®), a synthetic fluoroquinolone, represents a spectrum of broad antibacterial activity against Gram-positive, Gram-negative bacteria, mycobacterial organisms and anaerobes. Because of the presence of an asymmetric carbon atom in the molecular structure, racemic ofloxacin consists of equal amount of two enantiomers, (S)-(-)- and (R)-(+)-forms. Owing to the advancement of technologies for the synthesis and the separation of chiral compounds, (S)-(-)-enantiomer (levofloxacin) of ofloxacin was produced efficiently. Levofloxacin, was twice as potent as ofloxacin in efficacy while adverse events were significantly reduced. Since the first launch in Japan in 1993 as the first chiral fluoroquinolone antibacterial, levofloxacin is administered to 20 million patients every year. Levofloxacin is clinically recognized to be a powerful and safe weapon for the treatment of bacterial infections.
New horizon for cancer therapy
Leuprorelin (Leuplin®) was developed in Japan for the treatment of sex-hormone dependent cancers like prostate cancer. Continuous suppression of sex-hormone secretion is responsible for the therapeutic effect of leuprorelin. In contrast to previous available therapies for prostate cancer including female sex hormones and surgical castration, the quality of life is well maintained during leuprelin therapy. In addition, sustained-release dosing was achieved by microencapsulation in a biodegradable polymer. Gradual degradation of the microcapsules in the body results in controlled release of leuprorelin, allowing for up to four weeks therapy by a single injection. Leuprorelin has received approval in more than 50 countries worldwide.
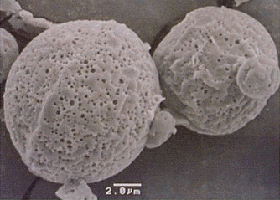
SEM micrograph of microcapsules
pGlu-His-Trp-Ser-Tyr-D-Leu-Leu-Arg-Pro-NHEt
Structure of the active ingredient of Leuplin®


